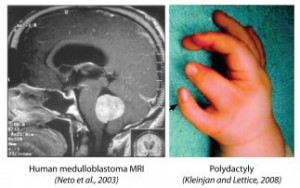
Hedgehog (Hh) signaling is one of the primary signal transduction pathways active in embryo development from fly to human. It is also important for many processes in the adult, including sustained generation of new neurons in the mammalian brain. The dysregulation of Hh signaling leads to developmental defects, such as polydactyly, and to cancer, such as the most common pediatric brain tumor – medulloblastoma.
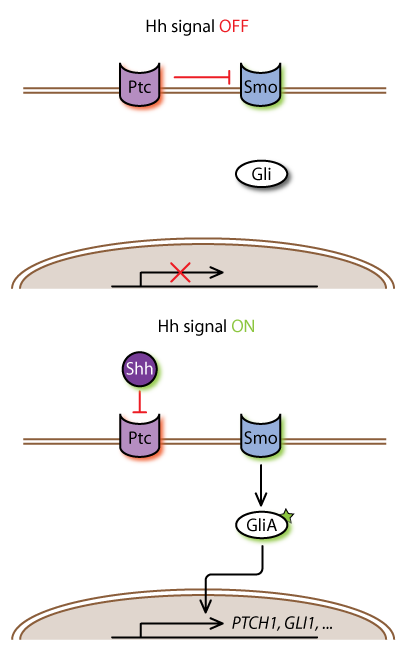
The Hh pathway is evolutionarily conserved and involves a number of steps beginning with the binding of the Hh ligand with its receptor Patched (Ptch). In its basal state Ptch represses the transmembrane protein Smoothened (Smo). Upon Hh binding, Ptch relieves its inhibition of Smo and then Smo transmits the signal downstream to a family of Ci/Gli transcription factors. The Gli proteins are a major focus of our investigation. Smo regulates posttranslational modifications of Gli2 and Gli3, and these in turn regulate the transcription of Gli1. Gli2/3 phosphorylation by protein kinase A (PKA) had long been known to induce the partial processing of these proteins into truncated forms which repress rather than activate transcription. We have recently shown that PKA phosphorylation, in addition to promoting Gli2/3 truncation, blocks the formation of transcriptionally active forms of full-length Gli2/3 . Smo inhibits phosphorylation of Gli2/3 by PKA, which results in the clearance of truncated Gli repressors and accumulation of Gli activators (see below).
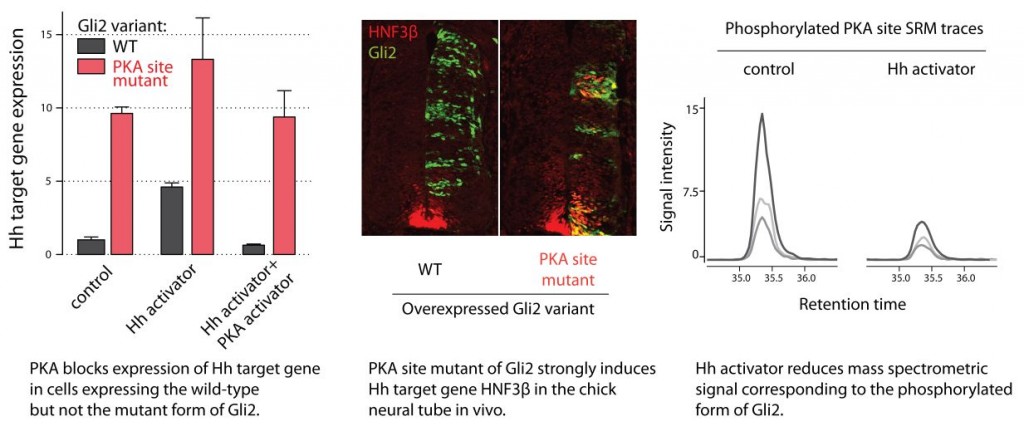 In addition to the canonical PKA target sites, Gli proteins are phosphorylated on many additional sites, some of which are specific for Gli activators, a phenomenon known as hyperphosphorylation. Neither the location of phosphorylated residues in hyperphosphorylated Gli proteins, nor the function of hyperphosphorylation are currently known.
In addition to the canonical PKA target sites, Gli proteins are phosphorylated on many additional sites, some of which are specific for Gli activators, a phenomenon known as hyperphosphorylation. Neither the location of phosphorylated residues in hyperphosphorylated Gli proteins, nor the function of hyperphosphorylation are currently known.
Questions that we are trying to resolve:
What sites participate in Gli hyperphosphorylation?
What is the role of hyperphosphorylation in Gli transcriptional activity?
What are the kinases involved?